The battery is the most important part of the machine, so maintaining the battery in good condition will be the key to effective engine starting, as well as the smooth operation of on-board electricity consumers. To properly operate the battery, you need to familiarize yourself with the basic principles of this device. This article will explain in detail how a car battery works.
What does a battery consist of?
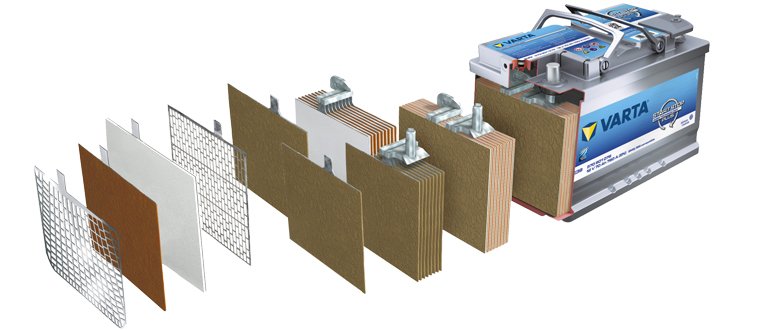
A car battery is assembled at the factory from many elements, therefore, to understand the principle of operation of an electric current source, you need to know the purpose of each component. The battery consists of the following parts.
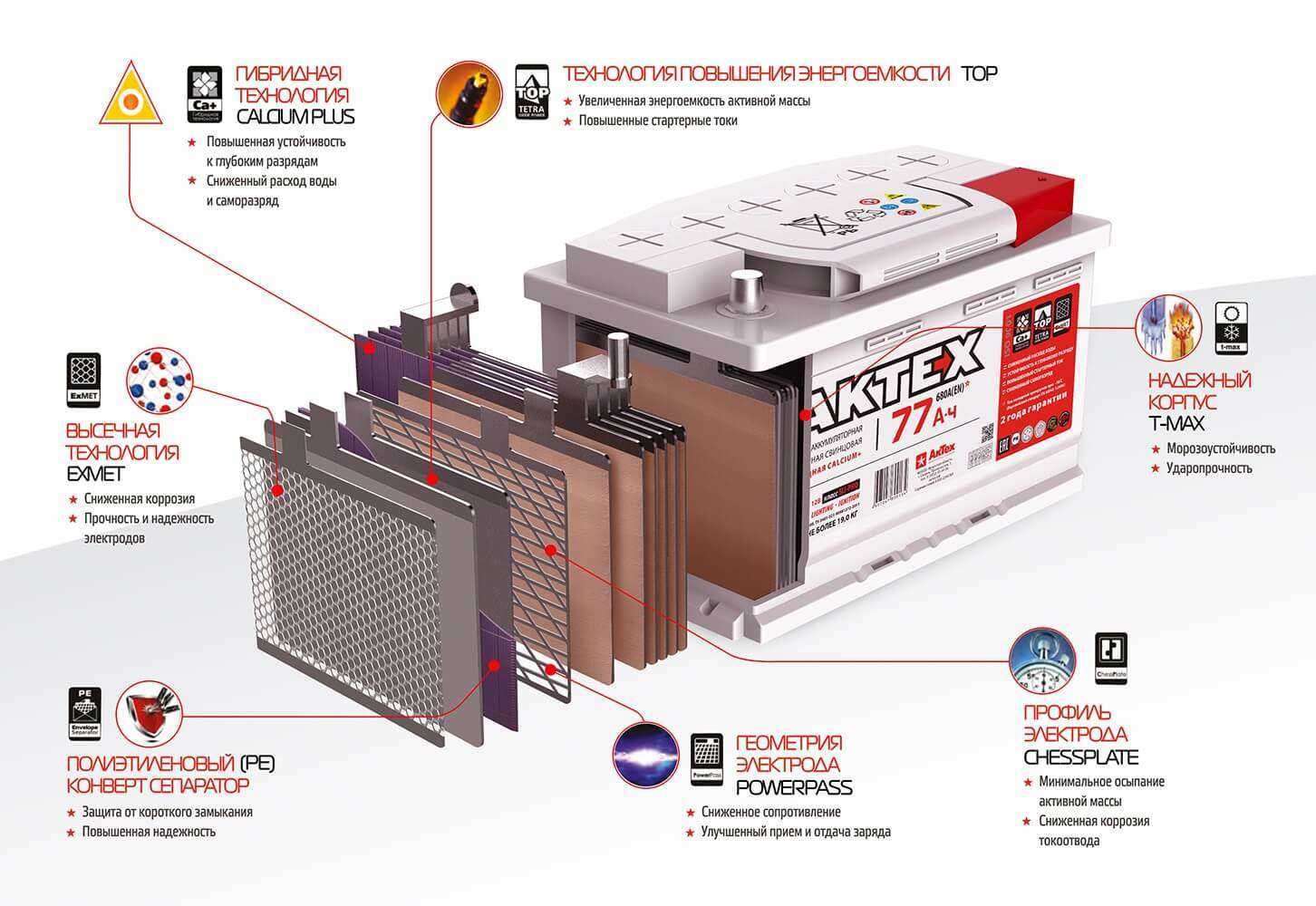
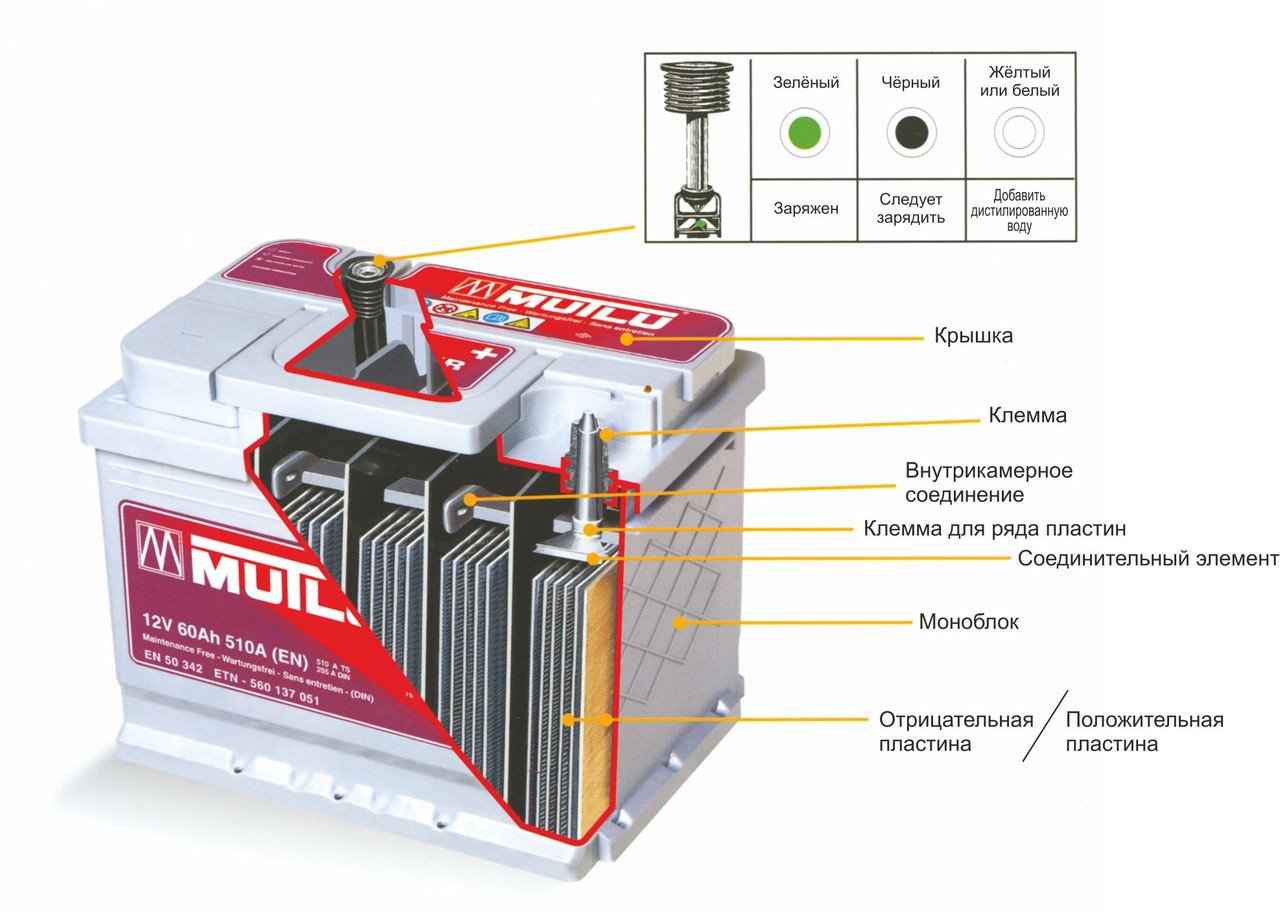
Body. Modern battery is made of high impact polypropylene. This material well tolerates not only increased mechanical stress and vibration, but is also resistant to acid, which in the form of a solution fills the internal cavity of the battery. In addition, polypropylene is resistant to large temperature differences. The battery case is divided into 6 hermetically separated sections, into which, in the process of manufacturing the battery, lead electrodes and separators are installed.
Separators Separators are installed between the electrodes and serve as dielectrics, which reliably protects the battery cells from short circuit. These elements are also made of acid-resistant polymer, which does not deteriorate when exposed to aggressive media during the entire life of the battery.
Electrodes Most manufactured batteries use lead plates with various impurities, in the cells of which there is a mass consisting of lead powder and sulfuric acid. The plates of modern batteries can be made of lead doped with calcium, which can significantly increase battery life.
Electrolyte. The electrolyte is a solution of sulfuric acid and distilled water. This fluid is necessary in order for the electric current to flow freely from negative to positive electrodes. In expensive batteries, instead of liquid electrolyte, it can be sealed using gel. Thanks to these qualities, gel batteries are available as completely maintenance free products.
Terminals All batteries have terminals, they can be of different types standard (European), ASIA (thin cones for Asian cars) and screw (for American cars). Occasionally, batteries with four terminals on the case can be met.
Additional functionality:
- For maintenance-free batteries, instead of the standard six plugs, there are 2 pressure relief valves on the sides (in case of electrolyte boiling, gas will be released through them).
- Some batteries are equipped with an “eye”, with which you can easily determine the degree of charge and electrolyte level.
How is the battery
The battery is designed in such a way that, as a result of applying direct current to its terminals, an effective accumulation of electrical energy occurs. Automobile battery consists of 6 containers isolated from each other, in which there are negative and positive plates, separated by separators.
Each such bank allows you to accumulate an electric current with voltage up to 2.1 V. To obtain the standard voltage of the car's on-board network, a series connection scheme for such electrical elements is used.An important feature of modern acid batteries is the complete sealing of the product body. Despite the impossibility of servicing this type of electric energy storage devices, their functionality and safety of use are at a higher level compared to products with plugs.
Battery Principle
A lead car battery is a renewable chemical battery in which electricity is generated as a result of a reaction between lead dioxide, sponge lead and a sulfuric acid solution.
When DC is applied to the battery terminals, pure lead is formed on the negative plates, and lead dioxide on the positive. When the battery is connected to various devices and units that consume electricity, the reverse process occurs, in which lead sulfate is formed on the negative electrodes and pure water is released from the electrolyte.
Depending on the type of battery, this sequence can be repeated thousands of times before sulphation or destruction of the plates occurs.
Design Features
Batteries may vary significantly. The design features of the battery include:
- Battery Size
- The composition of the metal alloy plates.
- Type of electrolyte.
- The location of the electrical leads on the housing.
The capacity of the battery will depend on the size of the plates and the amount of electrolyte in each bank, so the products installed to run the diesel installations of trucks can be several times the mass and volume of the battery for passenger cars.
The type of lead alloy will determine the internal electrical resistance of the battery and the resistance of the cell to aggressive environments. Also, the composition of the metal will affect the rate of moisture evaporation, therefore, for maintenance-free models, the plates are made of calcium doped lead.
A large number of battery parameters also depend on the type of electrolyte used in battery banks. The liquid solution freezes at low air temperatures, and when boiling leads to the evaporation of water, so replacing it with a gel can significantly increase the resource of products. Gel batteries can tolerate deep discharge much better, which allows them to be used not only as starting devices, but also for powering electrical power plants.
Batteries may also vary in terminal arrangement on the chassis. This parameter should be taken into account when selecting a new battery, otherwise it will be necessary to extend the positive cable of the car, connected to the power source.