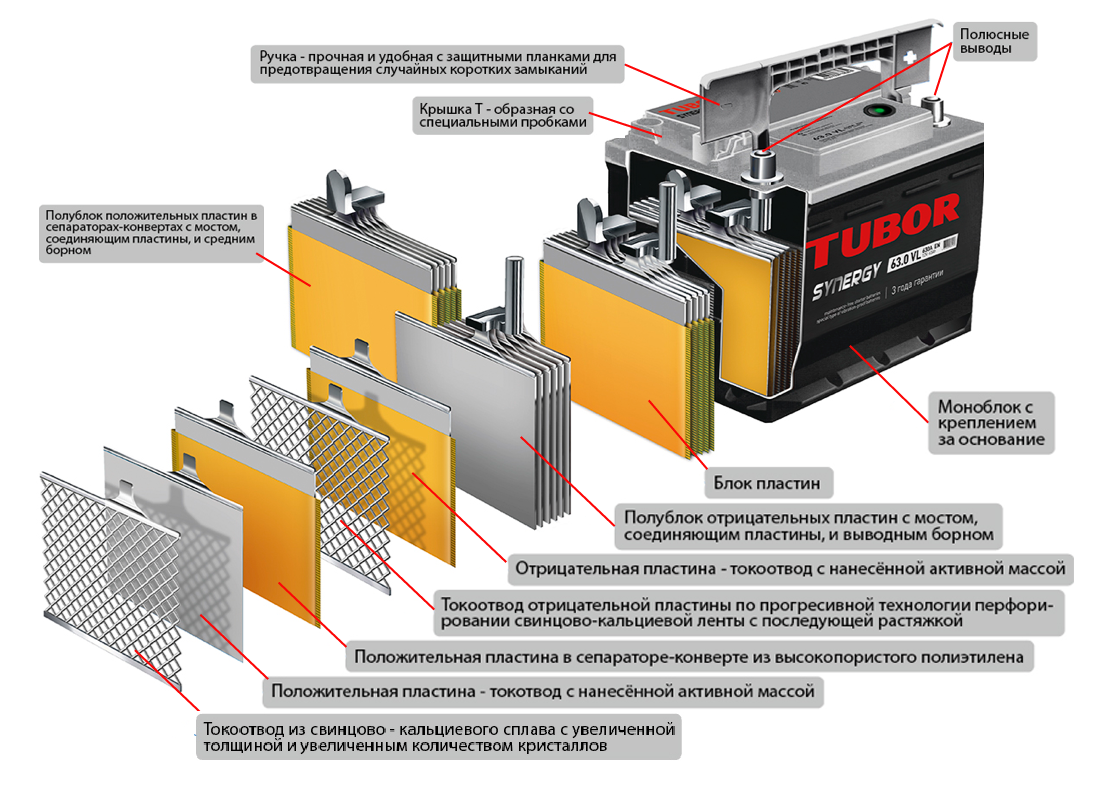
One of the most popular batteries in the world is still lead-acid. Despite the fact that this type of battery was invented in the 19th century, when its production began, due to its high efficiency it remains popular until now. Now you can find several varieties of such batteries.
Content
What is a lead acid battery
In the classic version, the battery consists of lead electrode plates, which are interspersed with a porous dielectric. This arrangement avoids circuit. The porous substance is called a separator. The whole structure is placed in an electrolyte.
In the modern design, the electrodes are made in the form of flat lattices of lead. Moreover, a powder made of lead dioxide is pressed into these lattices. Such an approach significantly increases the useful area of interaction of electrodes with electrolyte, so it is possible to increase the battery capacity.
The electrolyte is an aqueous solution of sulfuric acid. Only distilled water is used in the solution; there are no salts and mechanical particles in it, which can affect the operation of the battery.
An important parameter is the electrical conductivity of the electrolyte. This parameter depends on the percentage of acid in the solution, as well as on temperature. Conductivity corresponds to density; therefore, density measurement is usually used in practice. At room temperature, the optimum density of the electrolyte is considered to be 1.26 g / cm3, for this it is enough to get an acid solution in 35%.
Now you can find batteries that have instead of plates a network of interwoven carbon filaments coated with lead spraying. This allows you to reduce the weight of the battery, but at the same time increase the capacity. This technology is not cheap, which reduces its prevalence.
Sometimes a gel is used instead of liquid electrolyte. For this, the electrolyte is thickened with an alkaline solution of sodium silicates. This technology significantly extends the battery life.
Instead of a conventional separator, porous options made of fiberglass can be used. They are labeled - AGM. Allow hard charge / discharge modes.
Varieties and features of lead acid batteries
In practice, you can now find a variety of batteries. This allows you to solve a variety of tasks for the energy supply of a variety of devices. We will analyze the most popular types of batteries.
- Lead-acid Require maintenance. They are considered classic car starter batteries. This includes antimony, low antimony, hybrid and calcium acid batteries. Usually used for internal combustion engines, but this is not the only use case. There are options for 6v and 12v. The main disadvantage is the high level of self-discharge.
- AGM VRLA. Modern maintenance free batteries. They can have voltage - 2v, 4v, 6v and 12v. The main distinguishing feature - the separators are made of fiberglass. Absorbed electrolyte is also commonly used. This allows you to increase the battery life by one and a half times. The use of fiberglass separator technology has made it possible to increase the charging current, which speeds up charging. Usually AGM batteries It is allowed to charge with a current of 25-30% of capacity. Several varieties have been developed that are suitable for different buffer and cyclic modes.
- VRLA. Maintenance-free batteries with sealed housing (maintenance-free calcium and Efb) They can have voltage - 2V, 4V, 6V and 12V.Designed for operation in a buffer mode. Do not require additional ventilation when used indoors.
- GEL VLRA. One of the most modern modifications. A gel electrolyte is used here. A uniform consistency allows you to achieve the most effective contact of the electrolyte with the electrode, which increases the capacity. Also, the gel allows you to extend the battery life. It is a maintenance free battery. It requires periodic charging, to extend the life of the device you need to use only a high-precision charger, it must provide an accurate level of current strength. There are several varieties that differ in the characteristics of the electrodes. The designation of each of them allows you to determine the possibility of using the battery in certain conditions. There are 2v, 4v, 6v, 12v, 24v, 36v and 48v batteries.
- OPzV. This is a maintenance free battery 2V. They have tubular plates of electrodes. They are characterized by resistance to deep discharge and a service life of up to 22 years.
Even the basic varieties of lead-acid batteries are many, there are also subspecies. This allows you to choose the battery that is ideal for your needs.
Where are lead acid batteries used?
Such batteries are used quite widely. First of all, we are talking about the automotive industry, where all starter batteries are lead-acid. This use is due to the powerful current that they can reproduce, as well as resistance to a high level of discharge.
Also, it is often these batteries that provide stationary emergency sources. There is enough capacity to finish the job and put the equipment into off mode. An example of this are uninterruptible power supplies for computer equipment.
They can be used in the UPS in this case, they use batteries with the ability to give energy in small portions, but for a long time. Redundant power supplies have the same battery requirements. The best option for solving this problem will be AGM VRLA.
These batteries can be equipped with electric bicycles, gyro scooters, boat electric motors and other equipment. Here traction batteries are used that allow optimal and economical use of energy and can easily withstand frequent deep discharges.
General rules for charging lead acid batteries
Even sealed batteries require periodic recharging. Depending on the characteristics of operation, this action may be required, as after each working cycle, or once every six months or a year (car batteries).
Important! Current must be set to 10% of the nominal battery capacity. That is, if the capacity is 55 Ah, then the current should be no more than 5.5 amperes.
Capacity information is indicated directly on the battery case.
You can also often hear recommendations that it is required to open the covers. Modern sealed batteries do not require this. The lids have a special ventilation system, so there are no problems.
Be sure to charge at room temperature.
Batteries with an electrolyte gel can be immediately charged up to 20-30% of the capacity. It will not harm them. It is optimal for such batteries to use automatic chargers, which without your participation will adjust the required amperage.
Do not store lead-acid batteries in a discharged state. Therefore, be sure to charge the battery immediately after discharge, this will extend its life.